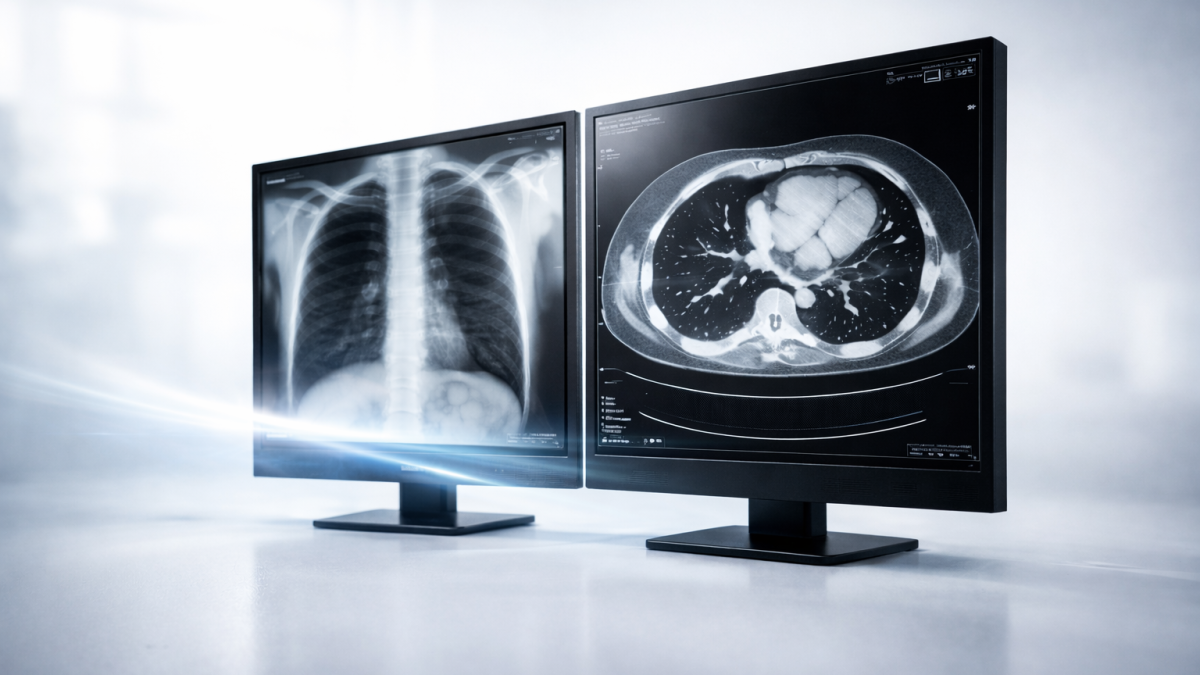
Beyond Standards: Defining Professional Medical Display Solutions: Atemitech Partners with Global Brands to Co-Create Diagnostic-Grade Terminals
February 10, 2026In medical device systems, the display module serves as a critical engineering node within the imaging chain. Factors such as grayscale reproduction, brightness stability, color consistency, and long-term performance directly impact the consistency and predictability of the system’s overall imaging. This, in turn, affects the integration efficiency for manufacturers during the design, verification, and mass production phases.
Atemitech specializes in the OEM / ODM / JDM development and production of Medical TDM (Touch Display Module) and professional display modules. From display engineering and electronic architecture to optical design, material selection, and manufacturing management, Atemitech provides integrated display solutions designed to support the system-level requirements of medical device manufacturers.
Positioning TDM: A System-Level Integrated Module, Not Just a Panel

The core of Atemitech’s TDM lies in system integration. Its design is engineered to meet the following technical demands:
- Foundation for Image Consistency: Designed in reference to professional display standards.
- Environmental Adaptability: Maintaining stable display performance under specific operating conditions.
- Development Convenience: Assisting clients in streamlining their end-device R&D and verification workflows.
Grayscale and Image Consistency: Engineering Based on DICOM Part 14
Medical imaging systems require high consistency and repeatability in grayscale reproduction. The Grayscale Standard Display Function (GSDF) defined by DICOM Part 14 provides a reference model for the relationship between digital values and displayed luminance. Manufacturers often incorporate this into their display design and testing protocols.
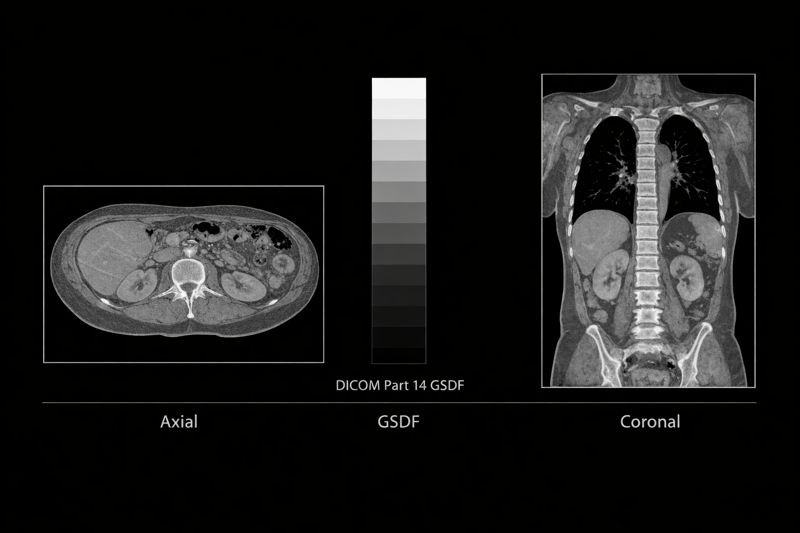
Atemitech incorporates DICOM Part 14 engineering concepts directly into the display module level through the following technical strategies:
- In-house Scaler Board Design: Proprietary image processing hardware.
- Hardware-Level Gamma Curve Calibration: Precise control at the circuit level.
- On-Module Parameter Pre-sets: Pre-configured color temperature and brightness settings
- Reduced Dependency: Minimizes reliance on the OS or external software for display behavior.
- Long-term Consistency: Enhances the stability of parameters over extended use.
- Simplified Validation: Streamlines system-level integration and testing processes.
Display Performance: Engineered for Clinical Efficacy, Not Just Specifications
1. High Color Gamut & Consistency
- High Chromaticity & Saturation Improvement: Ensuring vivid and accurate color reproduction.
- Chromatic Shift Control: Maintaining shifts within medically permissible ranges to ensure tissue boundaries and lesions remain identifiable from various viewing angles.
- Optimized Optical & Backlight Architecture: Significantly Reduced Light Leakage to prevent edge interference in dark-room diagnostics.
- Ultra-Wide Viewing Angles: Supports synchronized interpretation by multiple clinicians from different perspectives while minimizing luminance and contrast deviations.
- Stabilization Brightness Control (SBC): Maintaining constant luminance over time.
- Optical Bonding & Reflection Suppression: Eliminating air gaps to enhance contrast and readability in high-light environments while reducing visual fatigue for clinicians during extended use.


Why Atemitech is the Preferred Partner for Medical OEMs
- Regulatory Rigor: Design and manufacturing compliant with ISO 13485.
- Standard Compliance: Display systems meet IEC 60601 basic safety and essential performance requirements.
- Vertical Integration: In-house design reduces external dependencies and simplifies the validation process with Native DICOM Part 14.
- Supply Chain Stability: Medical-grade supply chain management ensuring long-term availability for device lifecycles.
Conclusion: Medical TDM as a Pillar of Clinical Trust
The value of Medical TDM lies in its predictability, verifiability, and long-term stability. For OEM / ODM / JDM clients, choosing Atemitech is not just a component selection—it is a strategic investment in medical quality and system reliability.
As the display module is not a medical device by law, the responsibility for regulatory compliance, certification, and legal accountability lies with the end-device manufacturer according to their product's classification.